|
The Science of Glow: Photoluminescence (aka UV Reactive) and Phosphorescence (aka Glow-in-the-Dark)
A very special thank you goes out to Daniel “Daf” Biner for his work on the contents of this section of the website. Daniel is a scientific technician at the University of Berne in Switzerland where he works in the field of inorganic chemistry. Most of his work is with light emitting materials. He is a self proclaimed kid who never grows up, a thrower of yoyos and painter of walls. Thanks Daf!
(pictured is Daf and his son Kris throwing yoyos) |
 |
Some simplified theory...
Photoluminescence is the process in which an object absorbs light, either visible or non-visible light, and gives off light of a different "color" (or "energy", as the scientific community likes to call it).
This is just one of many forms of luminescence (light emission). The time period between the absorption and the emission is usually very, very short, in the order of nano-seconds. (Think of a fluorescent yoyo string and yoyo under UV light).

Under certain conditions this time period can be extended to seconds, minutes or even hours. (Think about your GITD yoyo that keeps on glowing after you switch the UV lamp off)

The Phosphorescence-phenomena is a specific type of photoluminescence, related to fluorescence. A phosphorescent material simply re-emits the absorbed energy with a time-delay. This gain in duration is compensated by a loss in intensity.
This form of long-life luminescence should not be confused with other forms of long-life luminescence. A perfect example here is a glow stick, where the light emission is produced by a chemical reaction. This process is generally not reversible.
Common materials used to make stuff glow in the dark:
In the 1930's and 40's people used the radioactive element Radium to make the hands and dials of clocks glow in the dark. After the health risks of radiation were better understood this type of "paint" was wisely taken out of production.
This dangerous technology was initially replaced by zinc sulfide based compounds and mixtures. Unfortunately their performance was far weaker and would fade over time, therefore science called for better solutions.
These were provided by the discovery of Strontium Aluminates doped with Rare Earth Metals. These new compounds performed much better than zinc sulfide based recipes, and are found in our common GITD objects today.
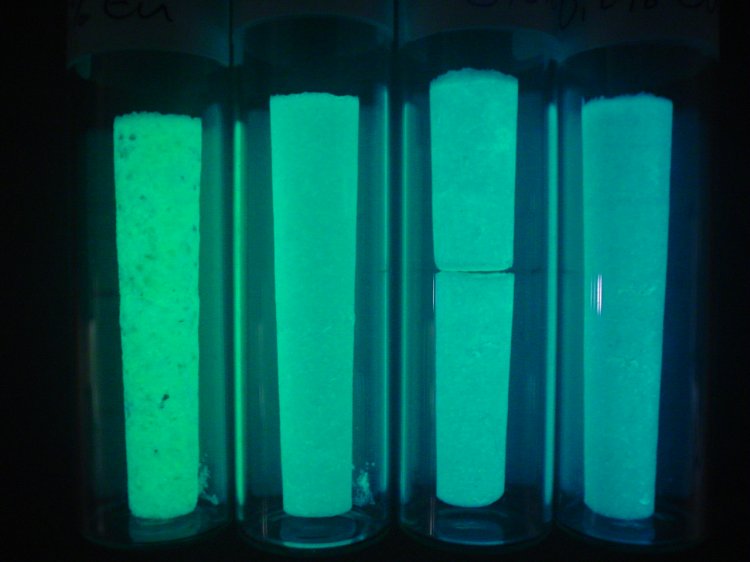
(Various samples of rare earth doped Strontium Aluminates)
Zinc sulfide based phosphorescence materials: Old Glow in the Dark Technology
Host: Zinc sulfide, ZnS
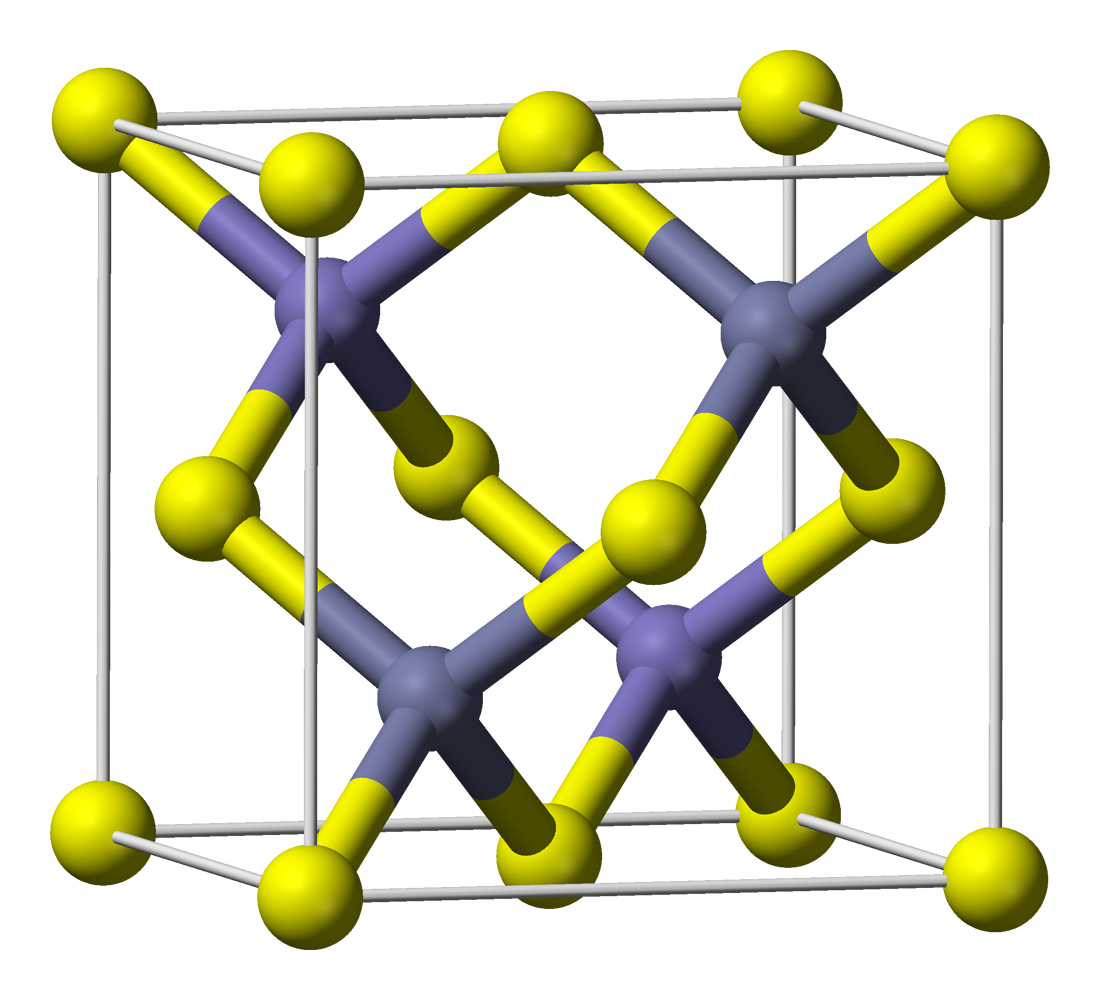
Pictured above is the common and more stable cubic form, known also as zinc blende. The yellow spheres indicate sulfur atoms, and the purple ones represent zinc atoms.
Active compositions:
The first phosphorescence using Zinc Sulfide compounds dates back to 1866. Many Zinc Sulfide compositions have been noted to show the phosphorescence phenomena, although in some cases the emission is comparably short-lived. Zinc Sulfide can be doped with other metals (also called "activators") resulting in various colors. For Example:
- doped with Silver: ZnS:Ag yields a blue glow
- doped with Manganese: ZnS:Mn yields an orange to red glow
- doped with Copper: ZnS:Cu is the most common composition and yields the longer lasting, well known yellow-greenish color.
Zinc Sulfide compositions still find applications in many low-cost GITD products.
Strontium aluminate based phosphorescence materials: New Glow in the Dark Technology
Hosts: Strontium aluminate compounds of different stoichiometric compositions, generally simplified to. SrAl2O4
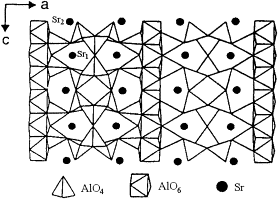
Pictured is one of the most commonly used, and probably the most efficient host material, Sr4 Al14 O24
The activators in these systems are typically rare-earth metals (lanthanides). The most common composition contains Dysprosium and Europium, giving off the well-known greenish-yellow luminescence.
The strontium aluminates are stable and odorless powders, mostly considered biologically and chemically inert. No health risks are associated with these compounds.
Doped strontium aluminates are superior phosphorescence materials to their predecessors, copper-activated zinc sulfides; they are about 10 times brighter and 10 times longer glowing, however about 10 times more expensive than the zinc sulfides.
They are frequently used in GITD toys, where they replace the cheaper but less efficient zinc sulfides. However, the material has high hardness, causing abrasion to the machinery handling it. When the plastics that contain strontium aluminates have to be machined (milling, drilling, turning on a lathe etc) the particles are often coated with a lubricant. But moreover, the plastics are only molded whenever possible, avoiding high costs in machinery.
Daniel "Daf" Biner
University of Berne
| 
